[ What is Life? ] [ Biomarkers ] [ The Importance of Oxygen ]
![]()
Biomarkers are features of a planet or atmosphere that are created through biological processes. These types of features are indicative of life, in the sense that the biomarker would not be present in the absence of life. Atmospheric biomarkers include oxygen, ozone, nitrous oxide, and methane. Water is often mentioned in this group due to the importance of its spectral features, but is not a true biomarker.
![]()
Oxygen is currently the most reliable biomarker due to its production by photosynthetic vegetation. Detectable levels of oxygen only accumulate through biological processes, like those seen here on Earth. Oxygen's main importance is derived from its production by plants. Once life began evolving, oxygen continued to accumulate in the atmosphere, indicating the prescence of life. In addition, oxygen is highly reactive, and so will only appear abundant in an atmosphere if it is continually replenished.
For more on the importance of oxygen as a biomarker, click here.
![]()
Ozone is a photolytic product of oxygen, which means that ozone is created through the chemical decomposition of oxygen by light. The more oxygen that is created by life, in turn, the more ozone that is created through the photolysis of oxygen in the atmosphere.
High concentrations of methane could indicate the presence of methanogenic bacteria or the decomposition of organic matter. However, an abundance of methane could also be produced by mid-ocean ridge volcanism. Yet, considering that methane is a highly-reduced gas, its presence in conjuction with the presence of a highly-oxidized species, such as oxygen, indicates processes other than atmospheric or geologic processes. Again, emphasizing the importance of oxygen as a biomarker.
Nitrous oxide is produced by life during microbrial oxidation-reduction reactions. This molecule is interesting because of its abundance due to biological processes, considering it occurs in only trace amounts from natural processes (Kaltenegger, Jucks, and Traub, 2005).
![]()
Water is highly esteemed because almost all life as we know it here on Earth requires water for survival. However, water is not a biomarker because it could exist on a planet without life necessarily being there. However, because all life as we know it requires water, its discovery on an exoplanet is the first step toward the discovery of life.
![]()
Water remains liquid over a wide range of temperatures.
This is important because the substance can remain liquid through changes in the weather or climate. The higher temperatures for water might also be necessary for life.
Ice Floats.
Most substances are denser when solid than when liquid, and so would sink. However, water is different. Since water ice floats, it helps life survive on Earth. In the winter, when surface temperatures are low enough for water to freeze, floating ice forms a layer of insulation on top of lakes and seas. This ice layer insulates the water below it, allowing it to stay liquid, which allows the life within it to survive. If ice sank, the liquid water on top would also freeze and sink, until all the liquid water became frozen.
 |
Water is less dense as a solid, than as a liquid, which is why ice floats. |
| Water is a polar molecule, with the oxygen having a slightly negative charge, and the hydrogens have a slightly positive charge. | 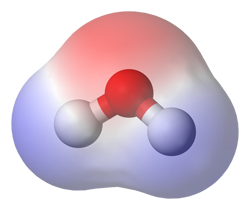 |
Water is a polar molecule.
The individual electrons within individual water molecules are distributed in a way that makes one side have a net positive charge and the other have a net negative charge. This charge separation affects the way in which water dissolves other substances. On Earth, the separation of water is critical to life. Living cells have membranes that do not dissolve in water, so the membranes effectively protect the interior contents of the cells. This separation also allows hydrogen bonds to be formed, which are important for the biochemistry of life on Earth as well.
Ultimately, water plays three vital roles for life on Earth:
- It dissolves organic molecules, making them available for chemical reactions within the cells.
- It allows for transport of chemicals into and out of cells.
- It is involved directly in many of the metabolic reactions that occur in cells.
![]()
Firstly, any liquid that would take the place of water must be abundant. On Earth, water is the most abundant liquid. In addition, the rate of a given chemical reaction drops in half for any 10°C drop in temperature. Water's high temperatures for its liquid state play a role in the fast reactions that occur in our bodies. Chemical reactions in ethane, which has a liquid range of -183°C to -89°C, would proceed much more slowly than in water. Any life using liquid ethane would have to have a much slower metabolism than organisms on Earth.